The Drug Supply Chain
The distribution of drugs from the drug manufacturer to the patient involves a variety of trading partners including drug manufacturers, wholesale distributors and dispensers. Drugs pass between these trading partners until they ultimately reach the dispensing unit and the patient.
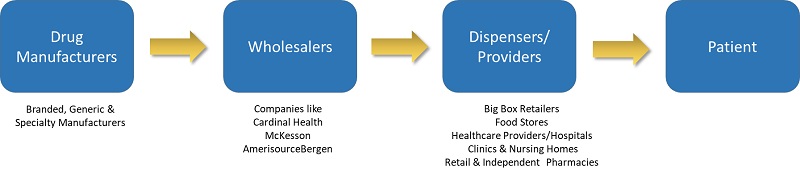
The drug supply chain has become increasingly complex as medicines and raw materials are being sourced throughout the world. Congress passed the DSCSA in 2013 that was intended to strengthen the US supply chain security by preventing counterfeit medications from being introduced. As the product moves down the supply chain, each company is required to exchange information about each drug every time a transfer of ownership occurs, creating a record of each trading partner’s ownership of the drug. In this way, the final point of sale has the complete pedigree of every unit. Drug Supply Chain Security Act (DSCSA) establishes requirements for the interoperable, electronic tracing of products at the package level.
Electronic Data Interchange and Automation
Electronic Data Interchange (EDI) is the most popular and secure method of electronically exchanging information between two trading partners. It has become an essential process for pharma manufacturers and drug wholesalers. Pharmaceutical companies need a robust EDI solution to comply with DSCSA requirements (pedigree reporting, expiration dates, and lot control etc.), to streamline order processing, to enhance communication with trading partners and reduce their overall time and cost.
Infocon Systems Cloud-based EDI solution can help with crucial e-pedigree and traceability requirements for Advance Shipment Notices (ASN) and inventory visibility that are now required by many pharmaceutical distributors and retailers, including Cardinal Health, McKesson, CVS, Walgreens etc. Infocon Systems takes EDI to the next level by integrating EDI documents into any ERP for its customers which immensely reduces the time and effort for keying and re-keying information into different systems.
Infocon Systems EDI Workflow
A retailer places a purchase order (EDI 850) on Infocon Systems EDI web-portal containing the quantities, product numbers, shipping instructions etc. which is then pushed via APIs or flat files into the supplier’s ERP system. In some cases, the pharmacy retailer can even see what the supplier has on hand with our inventory transparency. The supplier automatically sends a PO acknowledgement (EDI 855) and confirms the price from our web-portal. Once the order is placed, the supplier routes it to the warehouse/distribution center via their ERP to pick and pack the order. An Advance Ship Notice (EDI 856) with tracking details is pulled from the supplier’s shipping ERP and sent to the retailer. This is a crucial step that allows for receiving of the shipment through barcode scanning, resulting in faster unloading and sorting and the retailer can spot immediate loss and theft. The supplier creates an invoice (EDI 810) in their ERP/Accounting software and Infocon pulls it and sends it to the retailer via EDI. Infocon Systems’ algorithm automatically compares it to the PO, ASN and inventory packing lists to make sure everything matches and is approved for payment. If it doesn’t, the order is flagged for investigation.
For further information on how we can help you design your EDI workflow, you can live chat with our experts here. You can also email us at sales@infoconn.com or call us @+1-888-339-0722.